Structure of Atoms – MCQ’s
- Matter is composed of tiny indivisible particles called:
(a) Element
(b) Atom ✓
(c) Compound
(d) substance
- Atom of the same elements are
» View More
(b) Alike ✓
(c) Comparable
(d) Active
- Gas discharge tube experiment was performed by:
(a) J.J. Thomson
(b) Rutherford
(c) Dalton
(d) William Crooks ✓
- The pressure inside the discharge tube for the discovery of electron was kept
(a) 104 atm
(b) 10-4 atm ✓
(c) 1014 atm
(d) 10-14 atm
- Who was the pioneer of the vacuum tubes?
(a) William Crooks ✓
(b) Rutherford
(c) Bohr
(d) Dalton
- The nature of canal rays depends upon:
(a) Nature of anode
(b) Nature of Cathode
(c) Nature of gas ✓
(d) Nature of particles
- The mass of proton is ______ times more than that of an electron:
(a) 1830
(b) 1840 ✓
(c) 2
(d) 3
- Which one of the followings is produced by the bombardment of the helium particle on beryllium?
(a) Alpha particle
(b) Beta particle
(c) Neutron ✓
(d) Gamma rays
- The highly penetrating rays are:
(a) Alpha particle
(b) Beta particle
(c) Neutron ✓
(d) Both a & b
- Neutron was discovered by
(a) Rutherford
(b) Chadwick ✓
(c) Bohr
(d) William Crooks
- In -scattering experiment Rutherford used the foil made up of:
(a) Silver
(b) Tin
(c) Platinum
(d) Gold ✓
- Alpha particles are emitted by radioactive element:
(a) Carbon
(b) Polonium ✓
(c) Neon
(d) Vanadium
- Rutherford used the photographic plate coated with
(a) Zinc sulphide ✓
(b) Zinc sulphite
(c) Zinc oxide
(d) Zinc sulphate
- Who is the father of nuclear chemistry?
(a) Rutherford ✓
(b) Dalton
(c) William Crooks
(d) Joseph Proust
- According to quantum theory which type of spectrum is shown?
(a) Continuous spectrum
(b) Line spectrum ✓
(c) Emission spectrum
(d) Absorption spectrum
- Which of the following are fundamental particles of an atom?
(a) Ion
(b) Molecular ion
(c) Electron ✓
(d) Positron
- Matter is composed of tiny indivisible particles called:
(a) Ion
(b) Free radical
(c) Atoms ✓
(d) Molecules
- The meaning of Latin word ‘atom’ is:
(a) Chroma ✓
(b) Divisible
(c) Atomos
(d) Same place
- Rutherford used a thin sheet of gold of thickness:
(a) 0.00004cm ✓
(b) 0.004cm
(c) 0.0004cm
(d) 0.04cm
- Canal rays were discovered by:
(a) Goldstein ✓
(b) Thomson
(c) Dalton
(d) William Crooks
- Protons were discovered by:
(a) Thomson
(b) Chadwick
(c) Moseley
(d) Goldstein ✓
- Rutherford bombarded a thin sheet of gold with:
(a) a-particles ✓
(b) b-particles
(c) g-particles
(d) x-rays
- Which apparatus was used by Sir William Crooks in his experiment?
(a) Test tube
(b) Gas discharge tube ✓
(c) Zinc plate
(d) Electrolytic cell
- Which are three fundamental particles of an atom?
(a) Ion, radicals, free radicals
(b) Electrons, protons, neutrons ✓
(c) Electrons, protons, cathode rays
(d) Canal rays, x-rays, gamma rays
- The electrons revolve around the:
(a) Atom
(b) Nucleus ✓
(c) Protons
(d) Neutrons
- Nature of gas present in discharge tube affects the nature of:
(a) Canal rays ✓
(b) x-rays
(c) Cathode rays
(d) β-rays
- Alpha particles are basically nucleus of:
(a) Lithium
(b) Sodium
(c) Potassium
(d) Helium ✓
- Plum pudding model was put forwarded by:
(a) Dalton
(b) Thomson ✓
(c) Goldstein
(d) Chadwick
- Neil Bohr won the noble prize in:
(a) 1914
(b) 1918
(c) 1922 ✓
(d) 1926
- Canal rays travel in a straight line in a direction _______ to cathode rays.
(a) Opposite ✓
(b) Same
(c) Parallel
(d) None of these
- Rutherford, Moseley, Bohr and other scientists performed experiments and revealed that:
(a) Atom has complex nature
(b) Atom is neutral
(c) Atom can be divisible ✓
(d) Atom is beyond understanding
- Canal rays carry:
(a) +ve charge ✓
(b) –ve charge
(c) Neutral
(d) None of these
- Which isotope of carbon is in abundance?
(a) 12C ✓
(b) 13C
(c) 14C
(d) Both a and b
- Isotopes have different number of :
(a) Electron
(b) Proton
(c) Neutron ✓
(d) Charge
- Energy of N-shell is more than the energies of:
(a) K
(b) K, L
(c) K, L, M ✓
(d) L, M
- Quantum means:
(a) Fixed volume
(b) Fixed energy ✓
(c) Fixed pressure
(d) Fixed temperature
- The subshells of M-shell are:
(a) s, p
(b) s, p, d ✓
(c) s, p, d, f
(d) s, d, f
- N-shell can accommodate a maximum of ____ electrons.
(a) 8
(b) 2
(c) 18
(d) 32 ✓
- Which one is the electronic configuration of Cl-1?
(a) 1s2, 2s2, 2p6, 3s1
(b) 1s2, 2s2, 2p6, 3s2, 3p6 ✓
(c) Both a and b
(d) None of these
- Which one is the electronic configuration of sulphur?
(a) 1s2, 2s2, 2p6, 3s2, 3p6,
(b) 1s2, 2s2, 2p6, 3s2, 3p6
(c) 1s2, 2s2, 2p6, 3s2, 3p4✓
(d) 1s2, 2s2, 2p6, 3s2, 3p3
- The value of Planck’s constant is:
(a) 6.62×10-34 Js ✓
(b) 6.62×10-24 Js
(c) 6.62×10-19 Js
(d) 6.62×10-12 Js
- Which one of the followings results in the discovery of proton?
(a) Cathode rays
(b) Canal rays ✓
(c) x-rays
(d) Alpha rays
- The concept of orbit was introduced by:
(a) J.J. Thomson
(b) Rutherford
(c) Bohr ✓
(d) Planck
- Deutrium is used to make:
(a) Light water
(b) Heavy water ✓
(c) Soft water
(d) Hard water
- Co-60 is the source of:
(a) X-rays
(b) Beta radiations
(c) Alpha particles
(d) Gamma rays ✓
- For the production of cathode rays the pressure of gas inside the discharge tube was Kept:
(a) 10-1atm
(b) 10-2atm
(c) 10-4atm ✓
(d) 10-5atm
- Which one of the shells contains f–subshells?
(a) K
(b) L
(c) M
(d) N ✓
- Which one of the followings has only one neutron in its nucleus?
(a) Protium
(b) Deutrium ✓
(c) Tritium
(d) Helium
- Beta radiations are emitted by:
(a) Co-60
(b) C-12
(c) S-16
(d) Sr-90 ✓
- Rutherford won nobel prize in:
(a) 1909
(b) 1906
(c) 1908 ✓
(d) 1910
(a) 10 p
(b) -10 e
(c) 10 n ✓
(d) 42 He
» View Less

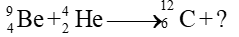
Great website for test session